It’s the start of flu season – time to protect yourself and your family by getting vaccinated! In today’s #FDA #SUNDAYTWEETORIAL I’m going to talk about #FDA’s role in ensuring the safety, effectiveness, and availability of the 2019 influenza vaccines. #VaccinesWork #FightFlu 

I know there are questions about the effectiveness and benefits of the flu vaccine given the severity of last year’s flu season, so I want to update you on what we’ve learned, the effectiveness of this year’s vaccine and why vaccination is important go.usa.gov/xPWhS. 

We've made important modifications to this year's #Flu vaccine to improve its effectiveness; changing two of the strains (for the H3N2 and Influenza B components) to better reflect the influenza strains that we believe are most likely to circulate this winter. More on this later.
First, some basics. Influenza, or the #Flu, is a contagious respiratory disease caused by different types of influenza viruses. Symptoms can include fever, headache, cough, sore throat, congestion and body aches. Complications of flu can be severe go.usa.gov/xPZqa. 
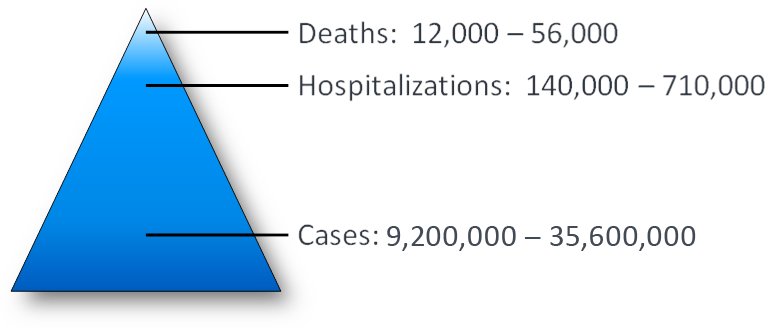
The @CDC estimates that the #Flu has caused between 9.2 million and 35.6 million illnesses; between 140,000 & 710,000 hospitalizations; and between 12,000 and 56,000 deaths annually since 2010. It now appears the numbers were even higher last flu season go.usa.gov/xPZaU. 
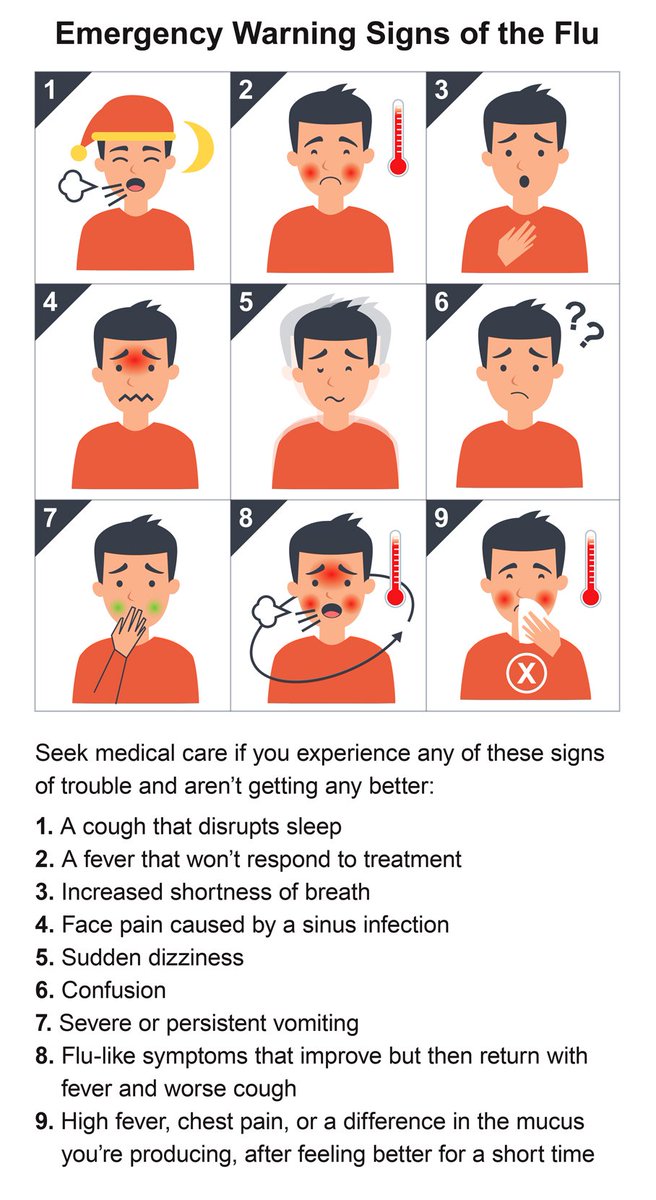
Children under five; adults over the age of 65, pregnant women, and people with certain health conditions are at higher risk of serious complications from the #Flu go.usa.gov/xPZqj.
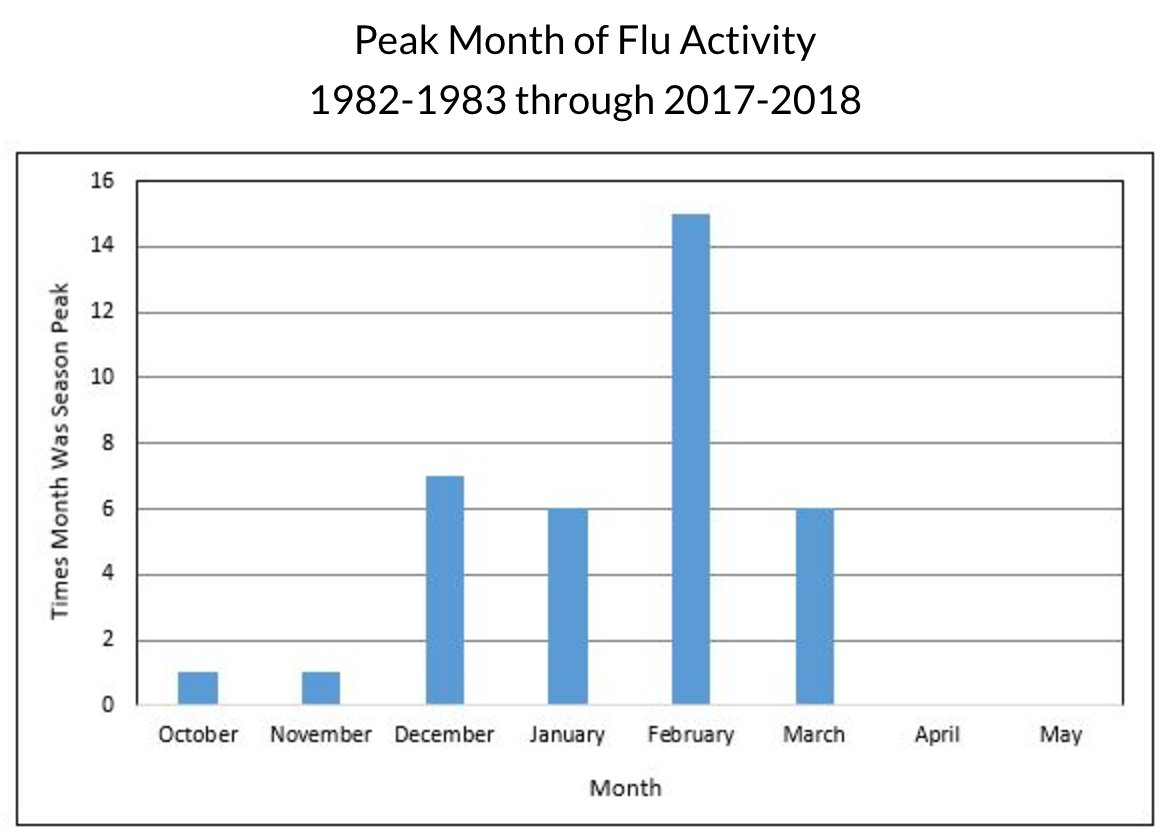
Seasonal #Flu viruses are detected year-round but are most common during the fall and winter. Activity often begins to increase in October and peaks between December and February, although activity can last as late as May. 
One of the most effective and safest ways to protect yourself and your family is to get the #Flu vaccine every year. FDA and @CDC recommend that most individuals 6 months of age and older get the flu vaccine to protect against influenza disease and its complications. 

How do #Flu vaccines prevent the disease? They expose the body’s immune system to versions of multiple flu strains that are anticipated to be circulating that season, so that the immune system is able to recognize and respond should the virus appear.
#FDA works year-round to ensure that the vaccines are safe, effective & manufactured to high quality standards. This includes evaluating & potentially adjusting the composition of the vaccine every year so that it matches the influenza virus strains expected to be in circulation 

Flu vaccines are designed to target the 3 or 4 of the flu viruses most likely to circulate during the season: 2 influenza A types (H1N1 and H3N2) and 1 (trivalent formulation) or 2 (quadrivalent formulation) types of influenza B. go.usa.gov/xPZYn go.usa.gov/xPZYU
FDA, WHO & @CDC review data on strains circulating worldwide to ID those likely to cause the most illness during flu season. Work is done months in advance to give time to make the vaccine. An FDA advisory committee chose this year’s strains in March 2018 go.usa.gov/xPZ4g. 
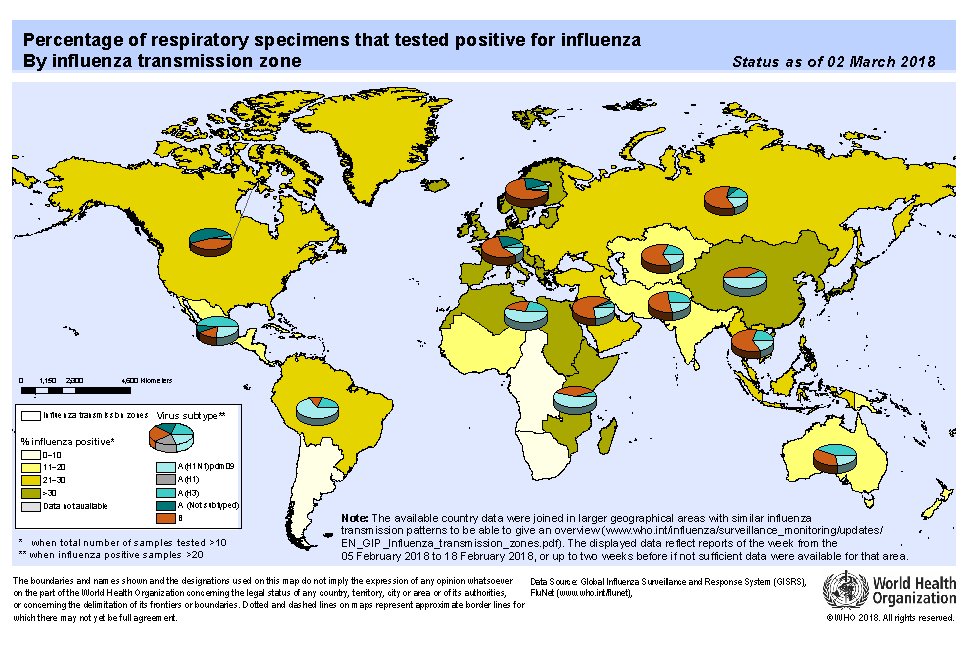
We’ve improved our scientific methods and changed some strains for this year’s vaccine. The #FDA has increased confidence, based on the pattern of influenza circulating now in the Southern hemisphere, that this year’s flu vaccine should offer Americans good protection. 
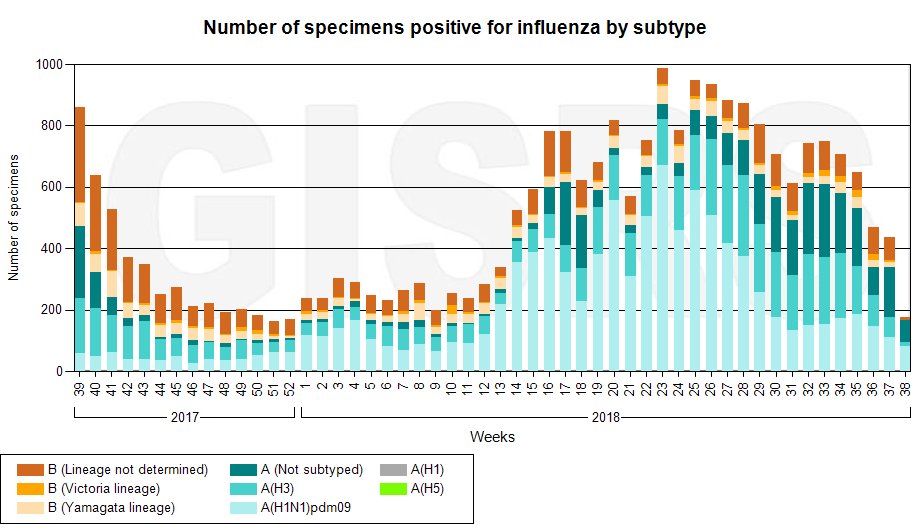
#FDA produces materials in our labs that are critical for making flu vaccines. We provide seed viruses used to make vaccine strains & reagents that are used to test vaccines for potency & identity. This ensures they are potent and includes the FDA recommended influenza strains. 

Throughout their manufacturing, the vaccines undergo quality control testing, including sterility testing. Manufacturers submit the test results and sample vials from each lot to FDA for lot release. Once #FDA OK’s the lots, they're distributed nationwide go.usa.gov/xPZYk 

We collaborate with our federal partners year-round to develop new ways to collect and track vaccine effectiveness and better understand if different manufacturing processes have an impact on effectiveness, so that we can identify ways to improve the #Flu vaccine in the future.
Most #Flu vaccines are made using chicken eggs. In recent years, #FDA has approved influenza vaccines that utilize other manufacturing methods - including cell culture and recombinant DNA technology - that offer certain advantages over the egg based system go.usa.gov/xPZcf 

During the 2017-2018 flu season, a preliminary analysis of @CMSGov data indicated that cell-based vaccines appeared to be somewhat more effective in preventing #flu than egg-based vaccines. More data are needed to better understand these findings go.usa.gov/xPZYp.
#FDA is working to facilitate development of more effective cell lines that can be better scaled through advanced manufacturing technologies, and ways to increase vaccine yield in recombinant vaccine manufacturing. 

These manufacturing advances hold great promise – potentially, supply could more easily be ramped up on short notice to adapt to changes in #flu strains since influenza viruses can mutate not only between seasons, but during the course of a single season go.usa.gov/xPDAS.
Could a #flu vaccine that lasts many years be on the horizon? Universal vaccines have the potential to prevent disease caused by any flu virus. However, much more research and development work needs to be done to make this a reality bit.ly/2NNIe4Z. 
What can you do now to prevent the #flu? Be sure to wash your hands with soap and water, cover your nose and mouth when you cough or sneeze, stay home when you’re sick and be sure to get your flu vaccine, like I did last week with the @Surgeon_General! It’s widely available now. 

• • •
Missing some Tweet in this thread? You can try to
force a refresh