Okay. Malam ni kita bukak topic on #Distillation #column process, bagi menjawab soalan @shahrinlatif98 

Okay. Certain #Distillation process, dia ada certain Reflux ratio.
Reflux ratio is defined as the amount of Reflux back to the column (R) to the amount of distillate out from the Reflux drum (D)
Reflux ratio = R/D
Reflux ratio is defined as the amount of Reflux back to the column (R) to the amount of distillate out from the Reflux drum (D)
Reflux ratio = R/D
Contoh dalam kes ni, your Reflux ration will be
Reflux Ratio = Reflux/Overhead Product
Reflux Ratio = Reflux/Overhead Product
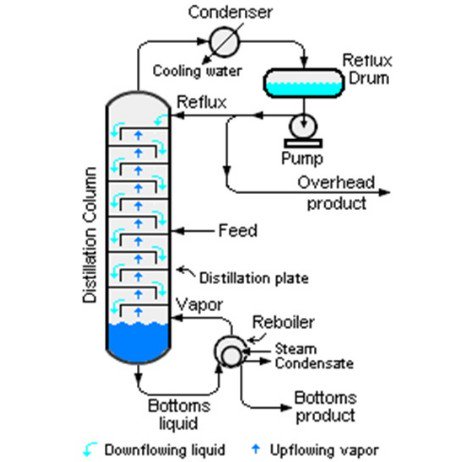
Kita sedia maklum, higher Reflux, leads to better separation. Number of trays needed pun become less
What if, we increase Reflux, R such that it approaches to infinity?
This will lead the Reflux ratio to also approach to infinity.
This condition is known as total Reflux
What if, we increase Reflux, R such that it approaches to infinity?
This will lead the Reflux ratio to also approach to infinity.
This condition is known as total Reflux
In other words, you only have Reflux, no distillate
Hence, Reflux ratio = infinity/0
Which is mathematically undefined
Hence, Reflux ratio = infinity/0
Which is mathematically undefined
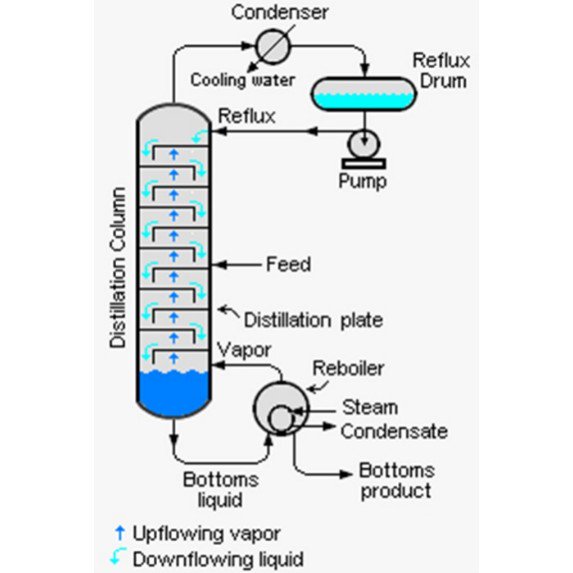
The only way to achieve this is to have an infinite reboiler heat duty and infinite condenser cooling duty
Which is not the case for our real world la kan
Which is not the case for our real world la kan
Kalau kita tak boleh nak achieve such condition (total Reflux), kenapa kita sibuk2 perlu tahu pasal benda ni?
🤔
🤔
Ingat, total Reflux adalah theoretical condition sahaja.
Dengan menggunakan Fenske Equation, at theoretical total Reflux condition, minimum number of theoretical stages can be determined.
Dengan menggunakan Fenske Equation, at theoretical total Reflux condition, minimum number of theoretical stages can be determined.
Ingat, kalau boleh, minimum kan number of stages for your #Distillation #column.
More stages means higher cost.
Kena bina column setinggi tinggi alam. Nak ke?
More stages means higher cost.
Kena bina column setinggi tinggi alam. Nak ke?
Higher capital cost satu, higher maintenance cost lagi satu.
Lagi banyak trays, means higher maintenance cost for tray repair activities
Lagi banyak trays, means higher maintenance cost for tray repair activities
Soalnya, kalau high Reflux ratio, operating cost lagi tinggi.
Kenapa? 😏
Kenapa? 😏
• • •
Missing some Tweet in this thread? You can try to
force a refresh